Backgrounds: Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative treatment for T-cell acute lymphoblastic leukemia/lymphoma (T-ALL/LBL). Relapse post-HSCT remains the major cause of mortality, highlighting the urgent need for more effective conditioning regimens in reducing relapse rates and improving outcomes for T-cell malignancy. Chidamide, as the first oral subtype-selective histone deacetylase inhibitor (HDACi) approved in China, can lead to a more relaxed chromatin structure through chromatin remodeling, particularly hyperacetylation of lysine residues in the histone tails, which facilitates the expression of tumor suppressor genes as well as the action of alkylating agents busulfan. To date, the role of chidamide in T-ALL/LBL conditioning regimens in allo-HSCT has not been explored.
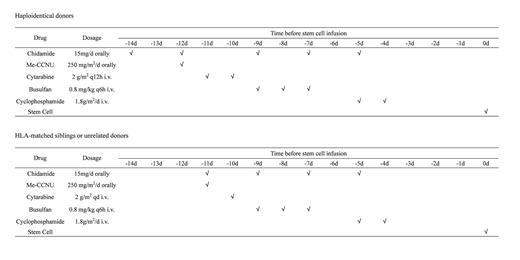
Methods: We retrospectively compared the clinical efficacy and safety between chidamide combined with a modified Busulfan-Cyclophosphamide (mBuCy) and mBuCy conditioning regimen for T-ALL/LBL patients undergoing allo-HSCT. The chidamide combined with mBuCy regimen was presented in Figure1.
Results: Twenty-two patients received chidamide combined with mBuCy conditioning regimen (Chi group). A matched-pair control (CON) group of 44 patients (matched 1:2) received mBuCy only. The median follow-up time was 683 (range 296-1168) days. Patients in the Chi group showed better 2-year overall survival (OS) and leukemia-free survival (LFS) (85.4 vs. 52.9%, respectively, P = 0.021; 69.1 vs. 42.9%, respectively, P = 0.031). Multivariate analysis showed that chidamide combined with mBuCy was a major positive risk factor for OS (HR 0.18, 95%CI, 0.05-0.63; P = 0.007) and LFS (HR 0.32; 95%CI, 0.13-0.80; P = 0.015). The cumulative incidence rates of grade II-IV and grade III-IV aGVHD were similar (36.4 vs. 38.6%, respectively, P = 0.858; 13.6 vs. 20.5%, respectively, P = 0.735). Patients in the Chi group showed higher incidence of elevated γ-glutamyltransferase and hemorrhagic cystitis than that in the mBuCy group (90.9 vs. 65.9%, respectively, P = 0.029; 36.4 vs. 13.6%, respectively, P = 0.007). No transplantation-related mortality was documented within the first 100 days after transplantation.
Conclusions: Our data demonstrate that the conditioning regimen containing chidamide improves the survival of T-ALL/LBL patients without increasing the incidence of transplant-related mortality. A prospective clinical trial is expected to confirm these findings.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal